13th AUGUST HAND - INTERNATIONAL ORGANS DAY
Dupuytren's Recurrence
A personal account of problems encountered
with Dupuytren's surgery and an
examination of the causes and avoidance
of subsequent recontracture
INTRODUCTION
I suffer from Dupuytren's contracture. It's a condition that afflicts mostly men in late middle age and results in their being unable to fully extend one or more of their fingers. The problem can be corrected with a simple operation but, despite the fact that all the material that needs to be excised lies no more than a few millimetres below the surface of the skin and its removal should be straightforward, patients can have very different experiences both in the short term recuperation phase and with long-term recontracture. In the following text, I will first describe the very different experiences I have had with my two hands. I will then cover some basic anatomy and follow this with a look at the histological reasons for recontracture. I will then identify one procedure that does give good results and which every sufferer of this complaint should know about. In creating this web site, I hope to aid others with Dupuytren's by helping them to avoid some of the problems that I have encountered.
Let me say at the outset, and make it clear, that I consider that I have been unfortunate to have encountered one surgeon who was plainly incompetent. I make no criticism of the large majority of surgeons who are conscientious and dedicated to the welfare of their patients. That being said, the appalling figures for the rate of recurrence of this complaint are not the result of the failings of just one surgeon.
MY EXPERIENCE
My right hand was the first to be affected and it was operated on 1997 with reasonable success. I didn't realize it at the time but I was lucky to be operated on by a surgeon who was interested in developing less invasive techniques and was, as a result, achieving much higher success rates.
In 2005, my left hand also became affected. Unfortunately, the surgeon who operated on me previously was not available and I was persuaded to go to another who, it was claimed, specialized in Dupuytren's. The fingers affected and their degree of contracture were identical to the condition of my right hand when it was operated on. So, not unreasonably, I assumed that this specialist would do at least as good a job as the first surgeon. However, five years after the operation, my left hand is now far worse than the condition it was in before surgery. In fact, it is so bad that further corrective surgery is unlikely to be successful.
My Right Hand
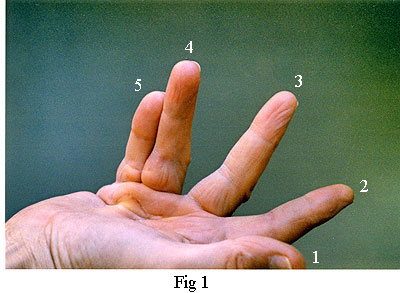
Fig 1, on the left, shows my right hand just prior to the operation. Note that the main contracture was in the fourth and fifth fingers. There was also partial contracture of the third finger. (It is the custom in the medical profession the identify fingers numerically 1 to 5 starting with the thumb and ending with the little finger).
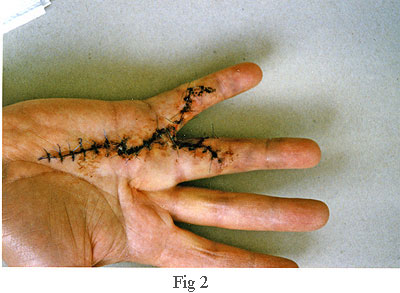 The picture on the right, fig 2, was taken about one weeks after the operation. The surgeon told me that he had not found the use of a splint (or cast) to be beneficial, so, he left the hand loosely wrapped in a bandage which allowed me free movement of my fingers. This encouraged blood flow which avoided clots and greatly accelerated the healing process. Another contributory factor to the healing was the perfect closure. In fact, I was very surprised that, apart from the dried blood visible along the line of the incision, there was no bleeding. Also, throughout the healing process, I lost no feeling or strength in my fingers. Looking back, I can honestly say that the recuperation phase hardly impacted on my life at all. When the time came to remove the stitches, about half of them had already fallen out because the healing process was so far advanced.
The picture on the right, fig 2, was taken about one weeks after the operation. The surgeon told me that he had not found the use of a splint (or cast) to be beneficial, so, he left the hand loosely wrapped in a bandage which allowed me free movement of my fingers. This encouraged blood flow which avoided clots and greatly accelerated the healing process. Another contributory factor to the healing was the perfect closure. In fact, I was very surprised that, apart from the dried blood visible along the line of the incision, there was no bleeding. Also, throughout the healing process, I lost no feeling or strength in my fingers. Looking back, I can honestly say that the recuperation phase hardly impacted on my life at all. When the time came to remove the stitches, about half of them had already fallen out because the healing process was so far advanced.

 The picture on the right, fig 2, was taken about one weeks after the operation. The surgeon told me that he had not found the use of a splint (or cast) to be beneficial, so, he left the hand loosely wrapped in a bandage which allowed me free movement of my fingers. This encouraged blood flow which avoided clots and greatly accelerated the healing process. Another contributory factor to the healing was the perfect closure. In fact, I was very surprised that, apart from the dried blood visible along the line of the incision, there was no bleeding. Also, throughout the healing process, I lost no feeling or strength in my fingers. Looking back, I can honestly say that the recuperation phase hardly impacted on my life at all. When the time came to remove the stitches, about half of them had already fallen out because the healing process was so far advanced.
The picture on the right, fig 2, was taken about one weeks after the operation. The surgeon told me that he had not found the use of a splint (or cast) to be beneficial, so, he left the hand loosely wrapped in a bandage which allowed me free movement of my fingers. This encouraged blood flow which avoided clots and greatly accelerated the healing process. Another contributory factor to the healing was the perfect closure. In fact, I was very surprised that, apart from the dried blood visible along the line of the incision, there was no bleeding. Also, throughout the healing process, I lost no feeling or strength in my fingers. Looking back, I can honestly say that the recuperation phase hardly impacted on my life at all. When the time came to remove the stitches, about half of them had already fallen out because the healing process was so far advanced.
My Left Hand
The surgeon who operated on my left hand showed no interest in the work done on my other hand. In fact, on each of three occasions when I questioned the necessity for a splint, he immediately changed the subject. He did believe in the beneficial use of a splint, no matter what the evidence to the contrary. So, when I came round from the anaesthetic, I found my left hand encased in a crude but very effective cast that held my fingers rigidly and fully extended. Another distinctive feature of the cast was that it was saturated with my blood. When I pointed out that I would have to keep the whole thing in a plastic bag to prevent the blood getting on my clothing, my protests were dismissed - indeed, it was all treated as a huge joke. However, it is noteworthy that, when I was leaving, a male nurse waylaid me and wrapped a bandage around the offending appendage, not to protect my clothing but rather to "...avoid frightening the people in the waiting room". Needless to say, wearing this cast for the next two weeks was no picnic; not just because of the inconvenience but also because it stank of stale blood every time it got damp. However, that was nothing compared with the shock I got when it was removed.
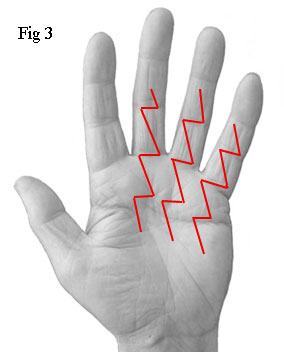 The first things I saw were three long zigzag incisions - not the single 8cm incision used by the first surgeon. In fact, the total length of these three incisions was a staggering 37cm (Fig 3). A separate incision had been made for each of the fourth and fifth fingers instead of the single incision used by the first surgeon. Also, he had operated on the third finger which was completely unnecessary. I'll explain the reason for this later. For the moment, note that the third finger of my right hand (see Fig 1) appears to be contracted. However, after the operation, it was straight despite the self evident fact that the surgeon had not operated on it (see Fig 2).
The first things I saw were three long zigzag incisions - not the single 8cm incision used by the first surgeon. In fact, the total length of these three incisions was a staggering 37cm (Fig 3). A separate incision had been made for each of the fourth and fifth fingers instead of the single incision used by the first surgeon. Also, he had operated on the third finger which was completely unnecessary. I'll explain the reason for this later. For the moment, note that the third finger of my right hand (see Fig 1) appears to be contracted. However, after the operation, it was straight despite the self evident fact that the surgeon had not operated on it (see Fig 2).
We then came to the business of removing the stitches. This would not have been such a problem if everything had not been caked with dried blood. I presume, in an attempt to lighten the increasingly tense atmosphere, the surgeon quipped that he would have to be careful as, in the past, he had been told off for not removing all the stitches. Watching him poking around in the mess that covered the palm of my hand, I did not find that at all surprising. I was, however, surprised that I could not feel the stitches being removed. Although the whole process with my right hand had been pain free, the one procedure that was quite painful was the removal of the remaining stitches. This was to be expected as I had never lost sensation in that hand during the healing process. I was now becoming very concerned that I couldn't feel anything over the palm of my left hand or up its fingers. I mentioned this at the time and on several occasions thereafter until, eventually, the surgeon gave the ill-tempered response, "of course your hand is going to be numb". In fact, there is absolutely no inevitability about this. Moreover, it is an ominous indication of trouble to come. Assuming no nerve damage, a lack of sensation is probably due to local ischaemia (inadequate blood flow), a condition that promotes fibroblast proliferation. I shall explain how this happens and the consequences later. Meanwhile, about three weeks after the stitches were removed, I found a stitch that the surgeon had missed. He had cut it off flush with the surface of the skin - the one thing that should be avoided.
In addition to the lack of sensation, I was quite unable to move my fingers. Their enclosure in the splint left them jammed in their fully extended position and nothing I could do would move them. This situation persisted for two or three days until, following constant manipulation, I was able to move them a little. It was seven months before I could touch the palm of my hand with the tips of the fingers. Over this period, there was also an almost complete loss of strength in the fingers. This condition slowly ameliorated over the following two years but, five years after the operation, I still have not regained the former strength in these fingers.
I have said that the first surgeon, who operated on my right hand, had closed the incision perfectly and that the healing was rapid. Unfortunately, the same cannot be said of the second surgeon. I think that I can reasonably claim that I have a strong stomach. I am not sickened by the sight of blood or injury. Certainly, when the splint was removed, it was not the sight of the congealed blood that coated my hand that disturbed me. What really appalled and nauseated me was the crude, amateurish closure and the fact that I could see very little evidence of healing. Instead of close and neat abutment of the edges of the incisions, there were numerous deep fissures and rucks (places where, between two stitches, one side of the incision lays flat whilst the other side is rucked up). Considering that, over the same period since the operation, the incision on my right hand had healed over, it was appalling that my left hand looked as if the operation had only just concluded. Later, after I had arrived home, my fears were borne out as all the wounds started to open up and suppurate. Unable to get help, I was obliged to try to hold the wounds together with strips of adhesive tape whilst a pad of lint retained the constant discharge of puss and blood. Adhesive straps were still in place when I returned to the surgeon about two weeks later. He made no comment about them and stated only, "Oh, that's fine". Now, it seems to me that there's nothing 'fine' about this and most professional surgeons would not think it 'fine' that their patients had to provide their own closure.
In view of the ample opportunity for infection during this early phase, I was paying particular attention for any signs of this. As the wounds finally started to heal over, I began to think that I was over the worst when, slowly, the whole hand started to swell up. At its worst, I could not move my fingers at all and, for about three weeks, I had to endure a constant throbbing pain until the swelling eventually abated.
The surgeon had always maintained that these small problems would be solved with plenty of vigorous massage and exercise. So, as soon as conditions allowed, he arranged for me to attend the physiotherapy department at my local community hospital. He said something to the effect that it would be all right as he had spoken with them. When I arrived, I was met by a young chap who seemed strangely reluctant to look at my hand. Instead, we just talked at length about the subject. When, eventually, I asked if he was going to start the treatment, he called his supervisor. This obviously irritated individual came in and immediately told me that the surgeon had visited their department to explain that the success rates for this operation were always going to be poor and, consequently, there was nothing that they could (or would) do. With that, the two of them turned and walked out of the room. Neither of them made any attempt to examine my hand. When I related these events to the surgeon, he was plainly annoyed, not that I had failed to received the treatment I needed but because he had failed to persuade that physiotherapy department to treat his patients. One can only surmise as to the full reasons for the physiotherapist's refusal to treat me but it was made very clear to me that they had made the decision not to treat the patients of that particular surgeon and they were not going to change their minds. Considering that they must have come to their decision after having had patients referred to them by that surgeon in the past, one wonders how long this has been going on and how many patients had been refused treatment? Also, just how bad does a surgeon have to be for a physiotherapy department to refuse to treat his patients. It is revealing to consider that all this was going on under the noses of National Health Service (NHS) executives, some of whom must have been involved as the people I met were unlikely to have acted in the way that they did without first getting the consent of their senior manager. One wonders just how many of the NHS management knew that there was a problem with that surgeon. I think someone should ask them why it was that the only action they took was to deprive their vulnerable patients of essential physiotherapy rather than address the actual cause of the problem.
There was nothing unique about my case. Mine was the most common form of Dupuytren's contracture. As I have already stated, the same fingers on both hands were contracted to the same degree. So, why is it that, while one surgeon can clearly demonstrate that it is possible to perform this operation with minimal recurrence, another surgeon, working on the same condition in the same hospital, is allowed to produce such grossly inferior work and continue to do so with impunity? Why is it that we patients are subject to this grotesque lottery?
Long Term Results
I stated in the introduction that the long term success rate for this operation is very poor. Taking some published figures[15], the following table gives a rough guide to the rates of recurrence:
| Years after Operation | Recurrence |
| 3 | 48% |
| 5 | 62% |
| 10 | 70% |
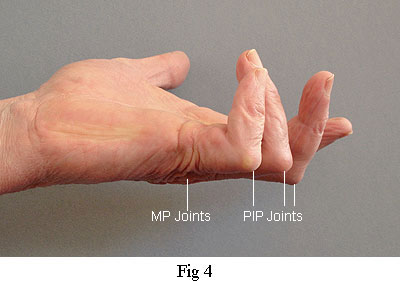
The figures in this table are also in close agreement with other surveys that I have seen. However, I suspect that the actual number of recurrences are even worse than these published figures. The definition of recurrence is usually taken to mean the reappearance of Dupuytren's tissue in the zone previously operated on. It does not include lesions that appear in adjacent and previously unaffected areas. So, according to this criterion, my left hand, shown below in Fig 4, has not suffered recurrence because the PIP joints were previously unaffected. Presumably, the people who collect these data would argue that the operation on that hand had been a success because the MP joints are still straight. As I shall explain, the excessive contracture of the PIP joints is most certainly due to the procedure used and the avoidable trauma of the operation. It also demonstrates how misleading these figures for recurrence can be. If the definition of recurrence took account of contracture caused by an operation to relive an adjacent joint, then the actual number of recurrences would be seen to be far worse than the already deplorable figures in the above table.
Also, considering that recovery takes most of the period leading to the point when another operation becomes necessary, the patient can find himself faced with the prospect of endlessly recuperating from botched operations and an ever diminishing likelihood of success. However, if, as with my right hand, the surgeon makes every effort to minimize the trauma, subsequent contracture, especially in adjacent joints, can be minimized. The following images illustrate the point.
The picture on the right was taken 13 years after the operation on my right hand. Although some recontracture has occurred, particularly in the PIP joint of the fifth (small) finger, at least it's not getting worse. Recurrence in the little finger is particularly difficult to avoid because of the small scale and unique ligament attachments that I will describe later. The picture on the left was taken just four years after the operation. Recontracture is severe and getting worse. It is worth reiterating that both my hands were in the same condition before they were operated on. That is to say, the same fingers were contracted to the same degree. The only difference was that they were operated on by different surgeons who used very different procedures.
Now, in order to understand why these procedures have such an influence on the outcome, I need to explain a little basic anatomy.
ANATOMY
A Few Terms and Definitions
The terms Proximal and Distal are widely used in anatomical reference. Proximal means 'nearer' or 'closer to some point or median line' and Distal means 'further' or 'furthest from some point or median line'.
Each of the three finger (or toe) bones is called a Phalanx (plural: Phalanges. Not Phalanxes: bodies of troops or police etc.). Of the three bones in each finger, the one nearest the palm is the Proximal Phalanx, then comes the Middle Phalanx and finally the Distal Phalanx. The thumb has just two Phalanges. The five Metacarpal bones lie between the fingers (and thumb) and the Carpus bones in the wrist.
Joints are identified as follows:
MP - metacarpophalangeal (Joint between metacarpal and proximal phalanx)
PIP - proximal inter-phalangeal (Nearest joint between two phalanx bones)
DIP - distal inter-phalangeal (Distant joint between two phalanx bones)
MP - metacarpophalangeal (Joint between metacarpal and proximal phalanx)
PIP - proximal inter-phalangeal (Nearest joint between two phalanx bones)
DIP - distal inter-phalangeal (Distant joint between two phalanx bones)
Collagen is a protein that is the principal constituent of white fibrous connective tissue. It is found in ligaments, tendons, cartilage, skin and bone. It is flexible with high tensile strength.
Please Note: in the following text, I shall use the term 'diseased' to refer to contracted cords made of collagen. It is a term widely used in medical circles when referring to tissue that is causing contraction which has to be excised to achieve extension of the fingers. However, as I shall describe later, the actual contractile tissue is not those bands of collagen that form part of the normal anatomy of the hand but rather fibrous material that has been deposited on them. I have seen no convincing evidence that the original anatomical bands of collagen become 'diseased' in the true meaning of that word.
Please Note: in the following text, I shall use the term 'diseased' to refer to contracted cords made of collagen. It is a term widely used in medical circles when referring to tissue that is causing contraction which has to be excised to achieve extension of the fingers. However, as I shall describe later, the actual contractile tissue is not those bands of collagen that form part of the normal anatomy of the hand but rather fibrous material that has been deposited on them. I have seen no convincing evidence that the original anatomical bands of collagen become 'diseased' in the true meaning of that word.
Fibrous Skeleton - In addition to the osseous (bony) skeleton, there is a skeleton of collagenous (made of collagen) fibres. It can be thought of as a flexible scaffolding that holds all the components of the hand, including the skin, in relation to one another whilst allowing considerable flexibility. The central region of the hand has three layers of these fibres: the Superficial Palmar Fascia which lies just beneath the skin of the palm, the Deep Palmar Fascia and the Dorsal Fascia which is on the back of the hand. It is generally the case that only the first of these, usually simply referred to as the Palma Fascia, is subject to Dupuytren's disease. In addition, there are groups of fibres that pass between each of these layers. The overall structure serves to hold the various components of the hand separate and in position when it is subject to the stresses of normal usage.
In the following diagrams, the main arteries are shown in red and the main nerves are shown in yellow. They tend to be grouped together in pairs called Neurovascular Bundles.
The Hand
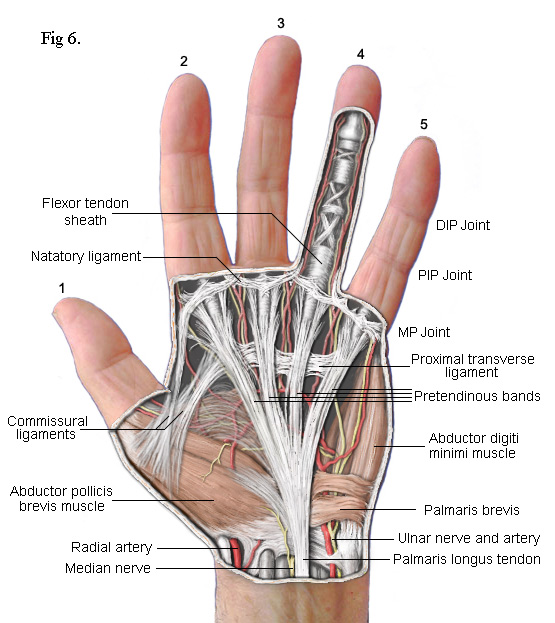 The drawing on the left shows the principal subcutaneous features. False colour is used to enhance clarity. The main feature is the part of the palmar fascia which consists of a series of fibres that pass up from the wrist and spread out, fan like, across the palm. These fibres bunch together into four groups called thePretendinous bands. Each band is aligned with one of the four fingers. As each bunch approaches the base of its respective finger, clusters of fibres break away to form separate connections. Most pass upwards to the skin where they form the cutaneous creases of the palm. Others pass down to a deep transverse metacarpal ligament (not shown) whilst others pass into the finger on either side of a central Flexor Tendon Sheath. Contracture of the MP joints is caused by Dupuytren's disease in these pretendinous bands.
The drawing on the left shows the principal subcutaneous features. False colour is used to enhance clarity. The main feature is the part of the palmar fascia which consists of a series of fibres that pass up from the wrist and spread out, fan like, across the palm. These fibres bunch together into four groups called thePretendinous bands. Each band is aligned with one of the four fingers. As each bunch approaches the base of its respective finger, clusters of fibres break away to form separate connections. Most pass upwards to the skin where they form the cutaneous creases of the palm. Others pass down to a deep transverse metacarpal ligament (not shown) whilst others pass into the finger on either side of a central Flexor Tendon Sheath. Contracture of the MP joints is caused by Dupuytren's disease in these pretendinous bands.
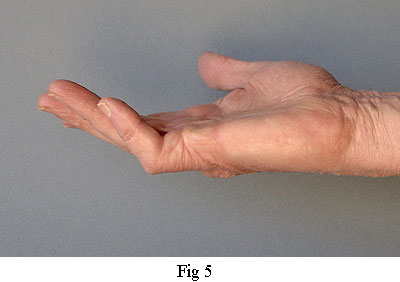
The palmar fascia also includes several collagenous transverse ligaments. Normally, these do not become diseased but one that does is the Natatory Ligament(from 'schwimmband' by Braune[2]). This ligament passes across the palm at the base of the fingers. Fibres within it pass from the Flexor Tendon Sheath of each finger into the lateral space of the adjacent fingers extending as far as the distal phalanx. When this ligament becomes diseased and contracts, it causes the fingers to be drawn together (see fingers 4 & 5 in Fig 1). It can also transmit stress from a diseased pretendinous cord of one finger to an adjacent finger. Thus, whilst a pretendinous cord will cause its respective finger to contract, the natatory ligament can also cause an adjacent finger to partially contract without there being any disease in its pretendinous band. The third finger of my right hand was affected in this way (Fig 1). When the surgeon released the pretendinous cord of its fourth finger, the third finger was also released. That, and the fact that I couldn't feel any pretendinous cord to the third finger, is how I know that the third finger of my left hand did not need to be operated on.
 The drawing on the left shows the principal subcutaneous features. False colour is used to enhance clarity. The main feature is the part of the palmar fascia which consists of a series of fibres that pass up from the wrist and spread out, fan like, across the palm. These fibres bunch together into four groups called thePretendinous bands. Each band is aligned with one of the four fingers. As each bunch approaches the base of its respective finger, clusters of fibres break away to form separate connections. Most pass upwards to the skin where they form the cutaneous creases of the palm. Others pass down to a deep transverse metacarpal ligament (not shown) whilst others pass into the finger on either side of a central Flexor Tendon Sheath. Contracture of the MP joints is caused by Dupuytren's disease in these pretendinous bands.
The drawing on the left shows the principal subcutaneous features. False colour is used to enhance clarity. The main feature is the part of the palmar fascia which consists of a series of fibres that pass up from the wrist and spread out, fan like, across the palm. These fibres bunch together into four groups called thePretendinous bands. Each band is aligned with one of the four fingers. As each bunch approaches the base of its respective finger, clusters of fibres break away to form separate connections. Most pass upwards to the skin where they form the cutaneous creases of the palm. Others pass down to a deep transverse metacarpal ligament (not shown) whilst others pass into the finger on either side of a central Flexor Tendon Sheath. Contracture of the MP joints is caused by Dupuytren's disease in these pretendinous bands.The palmar fascia also includes several collagenous transverse ligaments. Normally, these do not become diseased but one that does is the Natatory Ligament(from 'schwimmband' by Braune[2]). This ligament passes across the palm at the base of the fingers. Fibres within it pass from the Flexor Tendon Sheath of each finger into the lateral space of the adjacent fingers extending as far as the distal phalanx. When this ligament becomes diseased and contracts, it causes the fingers to be drawn together (see fingers 4 & 5 in Fig 1). It can also transmit stress from a diseased pretendinous cord of one finger to an adjacent finger. Thus, whilst a pretendinous cord will cause its respective finger to contract, the natatory ligament can also cause an adjacent finger to partially contract without there being any disease in its pretendinous band. The third finger of my right hand was affected in this way (Fig 1). When the surgeon released the pretendinous cord of its fourth finger, the third finger was also released. That, and the fact that I couldn't feel any pretendinous cord to the third finger, is how I know that the third finger of my left hand did not need to be operated on.
Note: The terms 'band' and 'cord' have specific meanings. In 1959, Luck[12] established the convention whereby normal fibrous tissue is referred to as 'band' and diseased tissue as 'cord'.
 Normally, the thin strands of collagen that make up the main components of the palmar fascia are separate. However, Dupuytren's disease causes these strands to become enveloped by new collagen into a single, much thicker, cord. So, numerous individual strands in a pretendinous band become a single thick cord. Within this cord are two distinct arrangements of fibrils. The linear form has fibrils arranged in densely compacted straight lines extending longitudinally along the cord and, embedded within it and superficially, there are small 'nodules' made of randomly arranged fibrils interspersed with cellular tissue. These give the cord a 'lumpy' appearance.
Normally, the thin strands of collagen that make up the main components of the palmar fascia are separate. However, Dupuytren's disease causes these strands to become enveloped by new collagen into a single, much thicker, cord. So, numerous individual strands in a pretendinous band become a single thick cord. Within this cord are two distinct arrangements of fibrils. The linear form has fibrils arranged in densely compacted straight lines extending longitudinally along the cord and, embedded within it and superficially, there are small 'nodules' made of randomly arranged fibrils interspersed with cellular tissue. These give the cord a 'lumpy' appearance.
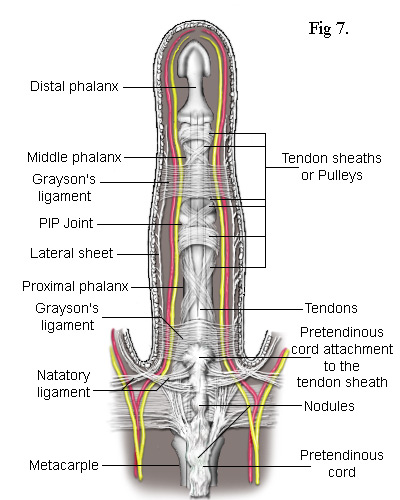
Central Cord - In a high proportion of cases, the pretendinous cord will follow a midline course and attach itself via a large nodule to the tendon sheath of the proximal phalanx (Fig 7). Thus, contraction of the pretendinous cord prevents the proximal phalanx from fully extending. In some cases, this cord will extend further along the midline to the middle phalanx attaching itself to its tendon sheath and contracting the PIP joint. Many strands of the normal pretendinous band pass upwards to the cutaneous layers where they are responsible for the creases in the palm. So, when this band becomes infected, deep folds and indentations develop in the skin. At the same time, the contracting cord becomes substantially thicker and forms a prominent palmer ridge in line with the finger (Fig 1).
As the cord is mostly superficial, its surgical extraction (fasciotomy) is fairly simple. However, the developing cord also invades the subdermal layers along its entire length and has to be careful separated. This inevitably results in some loss of the fatty subdermis which risks compromising the viability of the skin. The neurovascular bundles are relatively unaffected but, when the central cord combines with a lateral cord (a diseased lateral sheet), the neurovascular bundle can be displaced towards the midline putting it at risk of damage during surgery. There are numerous ways in which the pretendinous band, the natatory ligament, the lateral sheet and other ligaments in the finger can become individually or collectively diseased. The most common combinations of these are depicted in the following simplified diagrams. Figs 8 and 9 each contrast the unaffected lateral sheet on the left with the affected lateral cord on the right.
The Lateral Cord in Fig 8 proximally adheres to the natatory cord which also contracts preventing the adjacent finger from separating. The exception is the little finger where the natatory cord is also attached to the tendon of the abductor digiti minimi muscle (Fig 10). Another ligament to be involved is the Grayson's ligament[9] which, together with the Cleland's ligament (deeper and not shown), provides a conduit for the neurovascular bundle, securing it when a finger flexes. Cleland's ligament is much thicker and not usually affected by Dupuytren's disease but Grayson's ligament becomes enveloped by the spreading collagen and included into the lateral cord. So, as Grayson's ligament is attached to the tendon sheath at the base of the middle phalanx, when the whole cord contracts, it closes the finger at its PIP joint. This cord may also extend to the distal phalanx causing contracture of the DIP joint. Because the lateral sheet is normally inserted into the skin along most of its length, the lateral cord becomes deeply attached making its difficult to remove without damaging the subdermal layers.
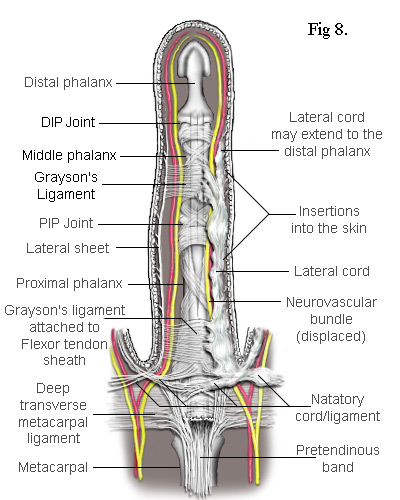
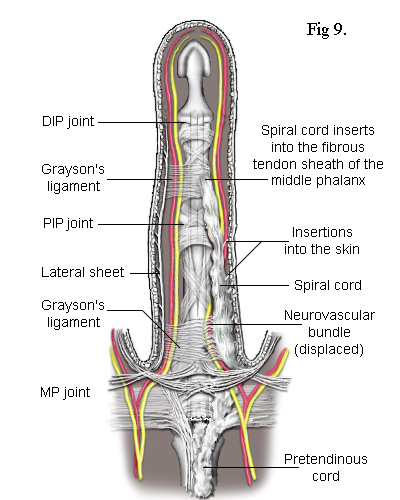
The Spiral Cord is similar to the lateral cord but differs in its proximal attachment. In the case shown in Fig 9, the attachment is to the pretendinous cord. Unlike the lateral cord, the spiral cord passes under the neurovascular bundle at the base of the finger and, as the cord straightens, this bundle is forced to spiral around it. As can be seen, the neurovascular bundles are displaced by both the lateral and spiral cords. In the latter case, the bundle is also pushed superficially making it particularly vulnerable to damage during surgery.
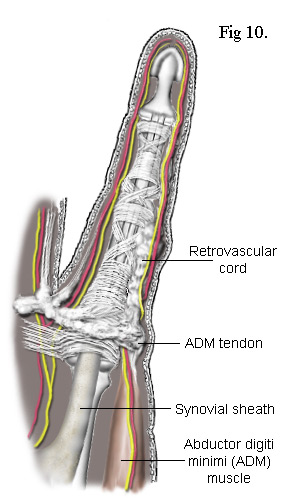 There are also Retrovascular Bands that can become diseased. I've not shown them in the above sketches partly because they are located behind the neurovascular bundles - hence their name. Like the spiral cords, they originate from the pretendinous cords. Passing laterally up the finger, they form connections with the natatory ligament, the proximal phalanx, the proximal end of the middle phalanx and end up bonded intimately to the collateral ligaments of the DIP joint. However, on the ulna side (on the same side as the ulna bone in the forearm - i.e. opposite the thumb) of the little finger, the retrovascular cord is almost always connected to the tendon of the abductor digiti minimi (ADM) muscle - see Fig 10. As stated earlier, the little finger commonly suffers contracture resulting from attachment of the natatory ligament to the tendon of this muscle. The combined affect is to splay the finger laterally and rotate it at the same time. Added to this, as the disease invariably spreads into the retrovascular band, all the joints in the little finger can become contracted as well.
There are also Retrovascular Bands that can become diseased. I've not shown them in the above sketches partly because they are located behind the neurovascular bundles - hence their name. Like the spiral cords, they originate from the pretendinous cords. Passing laterally up the finger, they form connections with the natatory ligament, the proximal phalanx, the proximal end of the middle phalanx and end up bonded intimately to the collateral ligaments of the DIP joint. However, on the ulna side (on the same side as the ulna bone in the forearm - i.e. opposite the thumb) of the little finger, the retrovascular cord is almost always connected to the tendon of the abductor digiti minimi (ADM) muscle - see Fig 10. As stated earlier, the little finger commonly suffers contracture resulting from attachment of the natatory ligament to the tendon of this muscle. The combined affect is to splay the finger laterally and rotate it at the same time. Added to this, as the disease invariably spreads into the retrovascular band, all the joints in the little finger can become contracted as well.
If the lateral cord is also present, it too becomes attached to the tendon of the ADM muscle.
There is a lot of variability between patients. The spiral cord, for example, may not originate from the pretendinous cord but from fibres at a deeper level and spiral cords may combine with long central cords contracting MP and PIP joints. The number, arrangement and termination of the pretendinous cords varies considerably. Within the fingers, any combination of central, lateral, spiral and retrovascular cords can also exist to any degree of involvement.
Now, it is a surgical priority to quickly and safely locate the neurovascular bundles so as not to accidently damage them. To this end, there have developed over the years a wide verity of incisions that variously claim not only to facilitate this but also to give adequate exposure of the diseased tissue, reduce skin necrosis, avoid contractile scarring and so on. Apart from the first surgeon who operated on my right hand, all surgeons with whom I have spoken proposed to use the zigzag type Bruner[4] incision. This was first published back in 1951 but has retained its popularity with plastic surgeons as it allows skin lengthening and improves access. However, skin lengthening was certainly not a requirement in my case, partly because I had not left it too long before consulting a surgeon. Also, extending separate incisions from each finger into the palm creates a problem. The centre of the palm is poorly vascularized[5,15] (i.e. there is a paucity of arterial blood supply) resulting in a risk of skin necrosis at the tips and exacerbating a problem with blood clot dispersal which I shall describe more fully later. In other words, habitually applying some standard incision cannot possibly be in the patients best interests. It should go without saying that the incision should be minimal and adapted to the clinical conditions.
Also, before we leave the subject of anatomy, it is worth mentioning an interesting feature of the digital web space that can facilitate the quick and safe location of the neurovascular bundles.
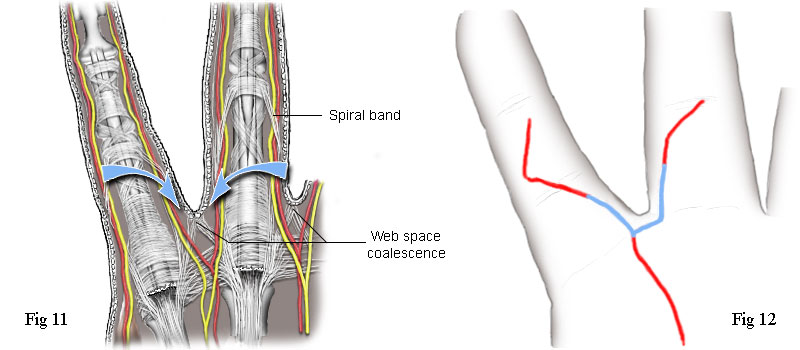 The web space is the soft tissue between the bases of the fingers (and toes). Some of the fibres of the pretendinous band, that pass either side of a flexor tendon sheath, pass below the neurovascular bundles and form a coalescence of fibres attached to the subdermal tissue directly between two fingers. See Fig 11. Immediately distal from this point and on either side of it (indicated by the two arrows), the skin does not adhere to the underlying fibrous tissue. Making an opening incision at this point enables the surgeon to quickly locate the neurovascular bundle and the fibrous cords that needs to be removed. Now, if we look again at the incision used by the first surgeon on my right hand, Fig 2, or the tracing of it at Fig 12, we can see the very significant fact that the incision goes directly over these two points. The place where the opening incision would have been made is shown in blue. Of course, I can't be absolutely sure that he was making use of this anatomical anomaly because I didn't think to ask him at the time. However, I now think it highly likely that he did. This type of incision gives good access, offers good insurance against accidentally damaging the neurovascular bundles, is far less invasive and is much more professional than to mindlessly repeat the Bruner incision irrespective of the clinical conditions.
The web space is the soft tissue between the bases of the fingers (and toes). Some of the fibres of the pretendinous band, that pass either side of a flexor tendon sheath, pass below the neurovascular bundles and form a coalescence of fibres attached to the subdermal tissue directly between two fingers. See Fig 11. Immediately distal from this point and on either side of it (indicated by the two arrows), the skin does not adhere to the underlying fibrous tissue. Making an opening incision at this point enables the surgeon to quickly locate the neurovascular bundle and the fibrous cords that needs to be removed. Now, if we look again at the incision used by the first surgeon on my right hand, Fig 2, or the tracing of it at Fig 12, we can see the very significant fact that the incision goes directly over these two points. The place where the opening incision would have been made is shown in blue. Of course, I can't be absolutely sure that he was making use of this anatomical anomaly because I didn't think to ask him at the time. However, I now think it highly likely that he did. This type of incision gives good access, offers good insurance against accidentally damaging the neurovascular bundles, is far less invasive and is much more professional than to mindlessly repeat the Bruner incision irrespective of the clinical conditions.
HISTOLOGY
Histology is the study of the microscopic structure of tissues - a subject in which I have a particular interest. As we have seen, collagen is a white, inelastic, fibrous constituent of 'connective tissue'. Connective tissue acts as a packing tissue or serves to separate organs. It consists of an amorphous 'ground substance' which can also contain elastic and reticular fibres (microscopic branching fibres that join together to form a supportive mesh for blood vessels, muscle fibres etc.), fat cells, mast cells (large cells that are a source of histamine, serotonin and heparin), macrophages (scavenger cells) and fibroblasts. Variations in the proportions of these constituents gives rise to the widely differing characteristics of connective tissue found throughout the body.
Collagen - Collagen is a major constituent of the body. It accounts for about 30% of the protein mass in mammals and is found in skin, bone, tendons, cartilage - even in the lens of the eye. It is divided into molecular types of which some 28 have been described in the technical press. Collagen found in connective tissue is usually of type I, II, III, V or XI. Dupuytren's contracture is mainly linked with just two of these - types I and III. Type I is the most abundant, accounting for 90% of all collagen in the body. It is very resistant to tensile (stretching) force. Type III gives structural support and is somewhat more elastic than type I. Importantly for this text, it is the principal constituent of granulation tissue which is created as part of the normal healing process.
Collagen does not have a cellular structure. It is protein. That is to say, it is a complex structure composed of many chains of amino acids, linked by peptide bonds. Typically, the basic component of collagen is composed of three strands of helical polypeptide chains wound around one another to form a triple-helical structure. When the body needs to create collagen - for example, as part of the healing process - it synthesizes these long molecules in cells called fibroblasts which then secrete and align these chains side by side to form fibrils of about 0.01 to 0.3 micron diameter (a 'micron' is one thousandth of a millimetre). The fibroblast can then arrange these fibrils and, given the right conditions, it will align them with other fibrils to create strong fibres of collagen up to 3 micron in diameter.
Fibroblasts - Apart from the precursors of collagen, fibroblasts also excrete elastic fibres and reticular fibres. The suffix '-blast' simply means 'active cell'. In stable conditions, these cells can switch to a less active state, in which case they are referred to as 'fibrocytes'.
fibroblasts play an essential role in wound healing. So, when tissue is damaged, fibrocytes become activated by growth factors and the resulting fibroblasts are induced to undergo mitosis (cell division) and migrate to the site of the wound.
Growth Factors - Early efforts to culture mammalian cells had only limited success. It was found that, when normal human tissue cells were cultured in standard conditions, only about 50 cell divisions would occur before they stopped dividing and died.
It was then found that, in addition to the usual nutrients - glucose, amino acids and vitamins - successful cell proliferation would only take place in a medium containing serum, the fluid derived from blood after it has clotted. It was soon shown that there are certain highly specific proteins within serum that, in very small quantities, will ensure continued cell proliferation. These proteins are called Growth Factors.
When blood clots, platelets within the clot are stimulated to release these growth factors from theirsecretory vesicles. One of the first to be identified was platelet-derived growth factor, orPDGF. We now know of about 50 growth factors. Within this range, there are families of factors, one of which is the fibroblast growth factor (FGF) family which has at least seven members.
One Important Conclusions should be Obvious from this:It is vital that blood clots are prevented from forming by encouraging the patient to exercise his hand. He cannot do this if his hand is fixed inside a splint. If a blood clot does form, a much higher concentration of growth factors will accumulate and disperse into surrounding tissue. This will produce elevated fibroblast activity. As a result, there will be greater collagen deposition in both the immediate and surrounding tissue. Even if the surgeon successfully removes diseased cords allowing one joint to be permanently extended, if the procedures he uses cause elevated fibroblast activity around the healthy ligaments connected to neighbouring joints, then those joints will undergo contracture within a few years - as happened to the PIP joints on the third, fourth and fifth fingers of my left hand.
|
Now, I've said that fibroblasts play a vital role in wound healing. Fibroblasts are stimulated into activity by the presence of growth factors that are released in the early stages of this process. Without growth factors, fibrocytes cannot be stimulated to become fibroblasts. With the prolonged presence of growth factors, fibroblasts cannot return to their dormant condition. Also, Dupuytren's cords are much thicker than the normal ligaments around which they form and, significantly, there are many reports in the literature that state that, when examined under the microscope, these 'diseased' cords look no different from normal collagenous tissue[3]. Such protein must have been created by normal fibroblast activity under the influence of growth factors even though no wound was present. It follows, therefore, that the root of the problem is the anomalous presence of growth factors in the subdermal tissue.
However, there remains the question of contracture. There must be some phase of the wound healing process in which fibroblasts exhibit the ability to contract the collagen that they have deposited.
However, there remains the question of contracture. There must be some phase of the wound healing process in which fibroblasts exhibit the ability to contract the collagen that they have deposited.
Wound Healing - Soon after injury to the skin, a complex series of events occur to stop the bleeding, close the wound and repair it. These events can be divided into four phases which are Haemostasis, Inflammatory, Proliferative and Maturation (Remodelling). The speed and degree of overlapping of these phases will vary depending on circumstances.
The bleeding is stopped (Haemostasis) by the enzyme thrombin acting on a soluble factor in blood called fibrinogen to produce an insoluble monomer (the basic component of a polymer chain) called fibrin. Fibrin then rapidly polymerizes (forms long molecular chains) to create a fibrous blood clot that seals off any damaged blood vessels and, ultimately, forms a scab.
In the Inflammatory phase, a range of scavenger cells called phagocytes engulf and digest bacteria, damaged cells and any potentially infectious material. Various factors included within the blood clot release growth factors that promote cell proliferation, stimulate inactive cells and induce cell migration. Because prolonged inflammation can lead to tissue damage, it is good clinical practice to minimize this phase by keeping the wound as clean as possible and avoiding slapdash surgical closure. This facilitates epithelial cells proliferation under the scab enabling closure of the wound with a new epidermal layer that excludes infection. There is good evidence to suggest that the continued presence of macrophages (a type of phagocyte) as a result of infection will inhibit subsequent phases [13] which, in effect, slows the healing process.
About two to three days after injury, the Proliferative phase starts in which an essential process called Angiogenesis occurs. This is the creation of new blood capillaries in developing granulation tissue. Throughout this and the following stages, it is essential that a good blood supply is established and maintained to bring oxygen and nutrients to the healing process. Limited hypoxia (oxygen deficiency) promotes growth factors and fibroblast proliferation. However, increased hypoxia will inhibit the fibrotic component of the provisional extracellular matrix, inhibit epithelial cell production and can lead to excessive fibrotic scarring. Also, hypoxia produces pronounced numbness. All of these symptoms were evident in the condition of my left hand when the splint was removed.
The development of fibrotic tissue is an essential process during the Proliferative phase as it helps to maintain the structural integrity of the wound and contract it. Initially, the fibrils (produced by fibroblasts) within this tissue are type III collagen and laid haphazardly as they connect to each other and to the edges of the wound. Some fibroblasts undergo differentiation into myofibroblasts which have some features that are similar to smooth muscle cells (hence the name - the prefix 'myo' denotes muscle). Myofibroblasts then migrate to the edges of the wound where they form linkages between the collagen fibrils and the edges of the wound. The myofibroblasts then pull on the fibrils which contracts the wound. Meanwhile, other fibroblasts reinforce the newly contracted wound with new fibrils. This process can take several weeks after which the myofibroblasts should undergo apoptosis (spontaneous cell death).
The development of fibrotic tissue is an essential process during the Proliferative phase as it helps to maintain the structural integrity of the wound and contract it. Initially, the fibrils (produced by fibroblasts) within this tissue are type III collagen and laid haphazardly as they connect to each other and to the edges of the wound. Some fibroblasts undergo differentiation into myofibroblasts which have some features that are similar to smooth muscle cells (hence the name - the prefix 'myo' denotes muscle). Myofibroblasts then migrate to the edges of the wound where they form linkages between the collagen fibrils and the edges of the wound. The myofibroblasts then pull on the fibrils which contracts the wound. Meanwhile, other fibroblasts reinforce the newly contracted wound with new fibrils. This process can take several weeks after which the myofibroblasts should undergo apoptosis (spontaneous cell death).
The Maturation phase can last for a year or more. During this phase, the original type III collagen fibrils degrade and are replaced by stronger Type I collagen. Also, the older disorganized placement of the fibrils is lost as fibroblasts secrete and align the new collagen in such a way as to oppose stresses being applied to the tissue.
Implications - This last point is very important as it refutes one of the main arguments employed to support the use of a splint during the period immediately following the operation. Every surgeon I questioned, who still insisted on fitting a splint, claimed that allowing the patient to contract their fingers would result in permanent contracture. They maintained that, without a splint, patients would hold their fingers closed in an instinctive move to protect the area of the incision. Also, when sleeping, the patient's fingers would naturally relax into a semi-closed position. There are numerous problems with these arguments. First, during this early phase of the healing process, the collagen being laid down is type III. It is, for the most part, disoriented and has limited strength. Physiotherapy and normal finger usage would easily overcome any constriction that it may cause - I know this from personal experience with my right hand. Dupuytren's recontracture does not occur during the Maturation phase. The rate of recontracture peaks three to four years after the operation. Maturation should be substantially complete between one to two years after the operation. So, although recontracture may well involve collagen that was deposited during the Maturation phase, it occurs long after the completion of the healing process.
Recontracture therefore involves original collagenous bands left by the surgeon that have become diseased (as in my case) and/or collagen fibres deposited during the Maturation phase that subsequently become diseased. Either way, it should be obvious that encasing the patient's hand in a splint will certainly not prevent recontracture. Quite the contrary, the conditions created by preventing movement of the fingers will greatly increase the probability of rapid and permanent recontracture after the removal of the cast. Preventing movement of the fingers inhibits blood flow and upsets the delicate sequence and timing of the phases of the healing process. It also prevents blood clots from being dispersed allowing growth factors to pool and collagen deposition to penetrate deep into tissue where normally it would not occur. For example, it is common for surgeons to find that they are unable to release recontracture, especially if it has advanced beyond 90º. In an investigation of seven amputated fingers severely contracted by Dupuytren's disease, Andrew[1]reported that extension of the PIP joints could only be achieved by releasing ligaments that hold the joints together - in particular, the collateral ligaments and the volar plate. Normally, these deep ligaments are not subject to Dupuytren's disease and would not prevent full extension during the first operation[15, P223-224]. However, there is a very high probability that they will become affected if there is prolonged recontracture after an operation. It would seen to be obvious that factors released during the healing process were implicated in the spread of the disease into the deep tissue and that making every effort to reduce trauma and expedite healing will minimize recontracture. Bearing in mind that Dupuytren's disease is caused by the anomalous deposition and contraction of collagen, it is surely perverse to follow an operation to remove this tissue by doing the very thing that will encourage its proliferation. Yet, by forcing the patient to wear a splint, that is exactly what happens.
I should also point out that two experienced surgeons have told me that they do not use splints because they have found that splints make no difference to the outcome. One has to ask why it is that some surgeons ignore the advice and experience of their knowledgeable colleagues and persist with procedures that defy science and common sense.
Getting back to the use of the Bruner incision mentioned in the anatomy section, another frequent justification for using this incision is that contractile tissue forming along a straight scar will inevitably cause recontracture. A zigzag incision, it is claimed, would allow surface tissue to stretch. Nice idea but not true! During the Maturation phase, type I collagen is laid through tissue that is subject to tensile stress - i.e. tissue that is frequently stretched. In both my hands, there is clear evidence that collagen fibres have been laid subdermally along straight lines that run from the fingers proximally across the palms. In other words, collagen has been laid in straight lines to oppose the stretching of the skin caused by extending the fingers. The deposited collagen does not follow the lines of the incisions. This would happen no matter what the pattern of the incision. Also, surface scar tissue does not by itself cause Dupuytren's recontracture and will, given time, diminish as the scar matures - a process that could take many years. However, there is a possibility that deeper developing Dupuytren's cords could invade the subdermal tissue and attach themselves to the scar tissue. In such cases, it is the cords that are securely terminated to, for example, the tendon sheaths that cause the recontracture, not the dermal scar tissue.
One of the problems confronting any surgeon attempting to release recontracted fingers is the amount of collagenous material that will have invaded the surrounding tissue. For example, by the time recontracture has advanced to the point where an operation is necessary, collagen will have invaded the subcutaneous fat. Trying to remove the cords can so damage the skin that it is no longer viable; in which case, a skin graft would be necessary. In other words, during the first operation, the diseased cords are clearly identifiable and their removal is usually straightforward. Subsequent operations to resolve recontracture are hampered by collagen that has spread into the skin, the deep tissue and the joints. Also, the previous surgeon could have left the neurovascular bundles displaced from their usual positions and they could be so enveloped in scar tissue that they are hard to identify. I shall return to the application of skin grafts as they offer significant advantages and the failure to inform patients of these has had serious consequences.
Another problem is the potential development of Sympathetic Reflex Dystrophy which manifests itself as pain and stiffness in the joints. It is rare. It occurs in about 5% of patients and is severe in about 1%. It isn't unique to Dupuytren's sufferers but it can occur if there are any complications. It is exacerbated by forced passive mobilization - in other words, aggressive physiotherapy. If the condition is aggravated by this means, the hand should be rested until the pain subsides when temperate mobilization should be started.
Finally, there is another aspect of dystrophy that brings us back to the questionable worth of splints. Every one that I questioned who advocated the use of a splint had insisted that the fingers should be fully extended. Yet, setting the fingers in a fully extended position is very likely to create sympathetic dystrophy and one doesn't have to do much reading to find statements such as this on the subject by Tubiana, Fahrer and McCullough[11]: "The posture of the hand during post operative immobilization must be in the anatomical position. Certainly extension of the metacarpophalangeal joints must be avoided." Now, I take this to mean that the hand should be in the relaxed position. It should not be set with the fingers fully extended as was the case with my left hand. One really does wonder if some surgeons who 'specialize' in this field ever bother to read the literature at all.
CAUSE
Much has been published regarding the nature and possible cause of Dupuytren's contracture. As stated previously, there are two quite distinct forms of Dupuytren's tissue - the cords and the nodules.
Microscopic examination has revealed no significant difference between the closely aligned collagen fibrils and fibres found in Dupuytren's cords and normally occurring collagen. In other words, there is no evidence to indicate that Dupuytren's contracture is caused by the denaturing or intrinsic changes in the length of the collagen fibrils per se[3]. Rather, the active stage of the disease is characterized by rapid synthesis of new collagen similar to that produced during the Proliferative and Maturation phases of the normal healing process. This indicates that Dupuytren's contracture is caused by a process similar to that of wound closure described earlier. However, instead of fibroblasts returning to their dormant state (fibrocytes) and myofibroblasts undergoing apoptosis, the anomalous continued presence of growth factors stimulates the deposition of repeated layers of collagen, each of which are then contracted by myofibroblast activity.
It has also been reported that Type III collagen, which is virtually absent in normal adult palmar fascia, is abundant in Dupuytren's tissue (10-20% in nodules and 30-40% in cords). Growth factors have also been found. Of these, Transforming Growth Factor beta (TGF-β), basic Fibroblast Growth Factor (bFGF) and PDGF have all been found to cause myofibroblasts proliferation but the former has created particular interest because of its ability to increase collagen production.
Gosset[8] and Hueston[10,11] have described the morphology and location of nodules. Isolated nodules adhere closely to the skin and are generally found in areas where no fibrous fascia structure is normally located. They tend to occur in areas where fatty tissue is thick, between interphalangeal creases or between the IP and MP creases. Nodules also occur on the anterior aspect (on the top) of the palmar fascia, never on its posterior aspect (underneath it). This would indicate that factors stimulating collagen growth are more prevalent in the subdermal fatty tissue.
It is now generally accepted that fibrous cords and nodules are two distinct forms of the disease that have developed in different tissues. The fibroblasts that create the fibrils of collagen have the ability to lay them alongside and bond them to extant bands of collagen, such as the pretendinous bands etc. Stimulated by growth factors, they recognize fibres of collagen and crawl along them, tugging newly secreted fibrils behind them to form a repeating staggered placement of successive fibrils linked by covalent bonds. The result is a thick and very compact fibre with considerable tensile strength. Alternatively, if the fibroblasts can find no pre-existing strands of collagen on which to align the new fibrils (as in fatty tissue) or if the regular staggered placement cannot be maintained, then the result is a nodule - a weak, disorderly agglomeration of loosely compacted fibrils interspersed with fatty cellular tissue that has been incorporated from the surrounding connective tissue.
Whereas previously it was thought that the body's own fibrous bands became diseased and underwent a fundamental change, it now appears that collagen thickly deposited on these bands is the actual source of the contracture. What stimulates this corruption of a process that normally serves so well to heal our physical injuries is still a subject for research. At least, I am unaware of any safe clinical treatment to stop it. However, if we cannot yet prevent this disease, there is certainly room for improvement in the methods used to correcting it. Apart from minimizing trauma during surgery, as already discussed, there is at least one surgical procedure that has a proven success record in reducing recontracture.
A BETTER WAY
As far back as 1952, Piulachs[14], and then Gordon in 1964[7] reported that there is no recurrence of contracture if the local skin is replaced by a free skin graft from elsewhere - a procedure called dermofasciectomy. One suggested reason for this is that there are fewer fibroblasts in the area from where the graft is taken - usually, the upper arm. However, the important point is that this fact has been known and verified[11,15] for years, yet none of the surgeons I consulted seemed to be aware of it. Only one surgeon expressed a willingness to do a dermofasciectomy but that was in the context of replacing damaged skin - not to avoid recurrence.
Every surgeon I consulted made a point of emphasizing the poor success rate of this operation. The physiotherapy department that refused to treat me had been told by the surgeon who operated on my left hand that a poor success rate was unavoidable. All the surveys of patients operated on for Dupuytren's contracture have concluded that the success rate is very poor. Yet, it now turns out that there is a surgical procedure that does give good results and a very low probability of recontracture under the area of the graft. Even if the full reasons for the prophylactic effect of skin grafts are not yet fully understood, there is absolutely no reason for not using this procedure to reduce the appalling rate of recontracture amongst Dupuytren's sufferers.
So, to take my left hand as an example, contracture of the PIP joints of the third, fourth and fifth fingers could have been avoided with a separate skin graft over the proximal phalanx of each finger. The point of this is that, even in the likely event that the surgeon would fail to remove all collagen fibres in these fingers that had the potential to become diseased, contracture of their PIP joints could have been avoided by removing the source of the growth factors that enabled myofibroblasts to develop. As removing the pretendinous cord is fairly straightforward and recontracture of the MP joints was unlikely, a palmar graft would probably serve little purpose, assuming the skin wasn't compromised.
Many patients will, I am sure, be concerned that the area from which the skin graft is taken will be scarred and unsightly. I can only say that if I had been given the choice of having straight fingers and such a scar or ending up as I have, then I would have unhesitatingly chosen dermofasciectomy.
For further reading on this subject, see ref 15, Pages 186 to 203.
Also, the following link may be useful:
Dermofasciectomy in the management of Dupuytren's disease. J.R. Armstrong, J.S. Logan, et al.
Dermofasciectomy in the management of Dupuytren's disease. J.R. Armstrong, J.S. Logan, et al.
COMMENT
Dupuytren's is not a trivial affliction. Unfortunately, many in the medical profession consider it to be a cosmetic problem. The truth is that almost every physical activity in which I engage is, to some degree, adversely affected by it. Everything such as household tasks, personal hygiene, driving, any work or leisure activity will be rendered more difficult or impossible by it. If you rely on playing a musical instrument to pay the bills, then that's the end of your career. I used to be able to type reasonably well - not any more. I'm typing this with two fingers. So, it is depressing to note that almost every surgeon with whom I have spoken has, in one way or another, asked whether I found it 'inconvenient'. One activity rendered almost impossible by this affliction is putting on a pair of gloves. As it happens, I use a lot of latex (surgical) gloves and I have found that putting them on is impossible unless I use the largest size obtainable. Even so, I can only tolerate them for short periods because of the pain caused by the pressure of thick folds of material that form at the finger joints. Any surgeon with this condition would have the same experience and would, I can assure you, find itvery 'inconvenient'.
Such are the problems encountered by people with this condition that, even being warned of the poor prospects, they would feel that they have little choice but to accept the risks. It is intolerable for victims of recontracture to then discover that the surgeon had failed to employ a proven procedure that substantially reduced this problem and that, as a result, they were in a far worse condition with little chance of improvement.
Because recontracture occurs three to four years after the operation, it is too easily dismissed as a separate issue. Yet, as I have explained, recontracture is substantially the result of procedures used - or not used - during the operation. If some surgeons are, for whatever reason, not using procedures that minimize recontracture, then steps should be taken to include the consequences of their inaction in the overall official figures for the success rate of this operation. In other words, four year follow-up examinations should be mandatory. At the moment, official figures give a wholly unrealistic picture of the true state of affairs because operations are considered to have been successful if the patient walks out of hospital with all five fingers intact and more or less straight. Then there is the question of cost. How much cheaper it would be, as well as beneficial to the patient, if surgeons were motivated to get it right first time. A more realistic approach would be to recognize the need to include recontracture avoidance as an important and necessary part of the operation and an assessment made to see that those aims had been met. In that way, surgeons with a poor record in avoiding recontracture could be identified instead of allowing them to continue, as at the moment, with total impunity.
I once asked a doctor if he knew the cause of this condition. He shrugged his shoulders and quoted some line from literature to the effect that these things just happen and we are subject to such vagaries as life will throw at us. I'm sure he was trying to inject a little light relief into a situation where he knew of many others who had experienced the same problems that I have just related. At any rate, I realized that he must be frustrated at seeing his patients end up as I did. However, if doctors are not in a position to improve the outcome of hand surgery, surgeons most certainly are. Unfortunately, there has developed a culture of reliance on excuses to explain away these poor results. I have been frequently told that even with the same patient, the same surgeon, the same technique and the same kit, the results of these operations are not predictable. In effect, these poor results are being shrugged off as one of those things that just happen - an act of God. It is a fact that "bad things" do not just happen. There is no capricious deity who, on a whim, decides that this operation will succeed whilst another will fail. Like it or not, it is a fact that the outcome of these operations is dependent solely on the procedures used and the professionalism of the surgeons.
Before concluding, I realize that there remains an un-answered question. It is obvious that the third finger of my left hand did not need to be operated on. Anyone with a basic knowledge of hand anatomy would have known that. So why did the surgeon bother to operate on it? Well, this troubled me for a long time until quite recently when, as part of the research for this web page, I contacted the BUPA hospital (now Spire Healthcare) where both operations took place. These hospitals have a system of fixed price contracts that they offer their patients. In other words, the patients are told that they would pay a fixed price even if there are complications or if further expenses are incurred. However, I have now discovered that this is not the whole truth. It seems that there was a scale of prices for Dupuytren’s surgery. For operations involving one or two fingers, there was one fixed price but, for three fingers, there was another significantly higher fixed price. Needless to say, I was not told about this at the time, either by the hospital or the surgeon. Thinking about it, this explains a lot. When the surgeon first examined my hand, he suddenly perked up and said “Oh, three fingers. I don’t often get a chance to operate on three fingers”. I thought at the time that he looked pleased at the prospect. Now I know why. Then there is the question of why he used three separate incisions when one would have sufficed and been clinically preferable. Perhaps he felt that it was more important to demonstrate that he had operated on all three fingers rather than use just one incision which would have been perfectly adequate to enable him to straighten all three fingers. Whatever his motives, they did not include due regard for his duty of care to his patient.
It is also worth noting that both surgeons who operated on me worked in the same buildings and must have known one another. Yet the successes of the first were no spur to the second to improve his results. Rather, he seemed to resent the first for taking his patients. When I tried to show the second surgeon my right hand, he contemptuously brushed it aside. It seems to me that if we patients could rely on those who operate on us to be motivated to learn from the successes of others, then, in all probability, my left hand would not be the abomination that it is today. Unfortunately, as the surveys and my experience proves, this ethos is not universal.
One ethos that is universal, however, is that which strongly inhibits every member of the medical profession from criticizing a confrère. To quote one surgeon whose facial expression clearly indicated that he disapproved of what had been done to my left hand: ”You can’t expect me to criticize this surgeon.” Actually, I do expect his criticism. In fact, it is largely because he, and the rest of his profession, can be relied upon to stay silent that we are now in this situation.
There is definitive truth in the axiom: No organization can self-regulate properly if it is incapable of self-criticism.
CONCLUSION
Low invasive procedures are much less likely to cause recontracture. The multiple joints in the hands (and feet) are particularly vulnerable to excess collagen deposition which causes recontracture. Every surgeon knows that the healing process creates collagen so it is hard to understand why so few hand surgeons are prepared to adopt procedures that limit its proliferation. I started this text by describing the relatively successful outcome of the operation on my right hand. As I explained, this result was due, in no small measure, to one surgeon’s endeavours to use less invasive techniques and expedite recovery. The results plainly show that his techniques did limit collagen deposition and were infinitely preferable to traditional procedures still widely used today. If your surgeon cannot explain how he proposes to limit collagen proliferation, then look for another surgeon.
If your surgeon tries to tell you that recontracture is inevitable, look for another surgeon. Although some contracture (10˚ or so) may well occur over a long period of, say, ten to fifteen years, there is absolutely no inevitably about the rapid and severe recontracture that has afflicted my left hand. That is the result of incompetence.
If your surgeon proposes to use a separate incision for each finger, as mine did, then look for another surgeon. That is beyond incompetence.
Dermofasciectomy is a powerful tool in the battle against Dupuytren’s recurrence. It is well documented in books and the technical press that recurrence very rarely occurs under a skin graft. Yet, depressingly, all of the surgeons I later consulted seemed to be unaware of this prophylactic effect and would only consider using it to replace damaged skin. Now, obviously, each patient is different and the merits of using this technique have to be assessed against other procedures when deciding what is best for the patient. However, no useful assessment can be made if dermofasciectomy is dismissed at the outset as a means to prevent Dupuytren's recurrence.
In cases where no skin graft is used, immobilizing the hand with a splint will serve no useful purpose. Impeding movement inhibits the healing process and the dispersal of blood clots that are a prolific source of growth factors. This actually encourages the re-growth of collagen that caused contracture in the first place. In cases where a skin graft is used, there is a requirement to hold it in place for the first seven days. To this end, a few surgeons have developed simple splints incorporating a pressure pad that allows some movement of the fingers. Under no circumstances should the fingers be set fully extended.
The natatory ligament can transmit stress from the contracted pretendinous cord of one finger to the adjacent finger making it partially contract. It does not follow that the pretendinous band to that adjacent finger is in need of excision. If a pretendinous cord is causing contracture of the MP joint of a finger, it creates a prominent ridge leading to that finger and is easily felt under the skin.
Repeated operations are much more difficult because of the widespread scar tissue and there is a far greater probability that they will make matters worse, especially if they don’t include a skin graft. It should be a surgical priority to GET IT RIGHT FIRST TIME.
ALTERNATIVE PROCEDURES
For completeness, I include the following. These procedures apply to anyone who has not yet been treated for Dupuytren's. Before considering surgery, you should be aware of them. I have little information other than what can be found by searching literature and the internet. Dupuytren's sufferers should ask their doctors for information about them as an alternative to surgery.
Needle Fasciotomy (aka: Needle Aponeurotomy)
This procedure was developed in France and evolved into a technique described by G. Foucher in 1999. It is normally only performed on a contracted pretendinous cord that is clearly defined. It involves inserting a needle under local anaesthetic and using the sharp edges of the needle to cut the pretendinous cord. The procedure is obviously far less invasive than surgery as the patient only suffers a few small holes made by the needle. However, there are a few serious problems that could arise. For example, if the needle is inserted too deeply, it could damage or sever the flexor tendons to the finger. The surgery needed to correct this mistake is made more complicated by the presence of the Dupuytren's diseased tissue. Then there is the need to avoid the neurovascular bundles. Sectioning cords in the fingers is hazardous as the cords can displace these bundles from their usual position. Also, this technique is never used after surgery because of unpredictable displacement of the neurovascular bundles by that surgery.
Recontracture is still a problem. In one survey[15, p129], it was reported that 54% suffered a recurrent lack of extension after an average of 2.5 years. Of all surveyed, 11% needed further surgical treatment. The problem is that the source of the growth factors that caused the collagen deposition which created the initial contracture has not been removed. Also, the cut ends of a severed cord will be quite close together, even with the finger fully extended. Whereas fibroblasts cannot rebuild a complete pretendinous band, it has been shown that they can bridge remarkably large gaps with new collagen. If the cut ends of the pretendinous cord become firmly reattached in this way, the contracture process will resume. One possible solution is to fully extend the finger with a splint worn every night. The reforming collagen must not be allowed to build to the point that it can prevent the full separation of the cut ends of the severed cord.
Injection Therapy
Proteins, like collagen, have enzymes that degrade them. They are usually given names that end in "ase", so the enzymes that degrades collagen are called "collagenases". They attack the matrix of the collagen fibres at selected points and destroy its integrity. It follows that, if collagenase is injected into a diseased cord, it will degrade and break under pressure to extend the finger. Although this does work, it can be very painful. Here again, a search of the internet is recommended.
Radiation Therapy
This is a promising technique that has the potential to slow the progress of the disease if used in its early stages. Like Needle Fasciotomy, most British patients remain ill-informed about these alternatives to surgery, all of which were pioneered in France and Germany. It's heartening to see web pages appearing from those who have sought out and been treated by these new and far less traumatic procedures.
The following link is a good example:
http://www.pushpullsigns.com/dupuytrensradiotherapy.html
The following link is a good example:
http://www.pushpullsigns.com/dupuytrensradiotherapy.html
I hope the information in this web site will encourage and equip patients to ask searching questions and enabled them to make informed decisions.
In the words of Enrico Fermi: "Ignorance is never better than knowledge".
R. Ashby
First Published: June 2010
Updated:
Left Hand Section - Extended. December 2010
Comment Section - Extended.
Conclusion Section - Extended and clarified.
Histology Section - Extended and clarified. April 2012
I intend to publish periodic updates.
First Published: June 2010
Updated:
Left Hand Section - Extended. December 2010
Comment Section - Extended.
Conclusion Section - Extended and clarified.
Histology Section - Extended and clarified. April 2012
I intend to publish periodic updates.
Links:
The British Dupuytren's Society is a quite new UK charity. Its web site is helpful and informative.

No comments:
Post a Comment